- The root-mean-square value of an alternating current is equivalent to the steady direct current that would dissipate heat at the same rate as the alternating current in a given resistor.
- For a sinusoidal source,
(a) the root mean square value of the current is given by
(b) the mean or average power < P > absorbed by a resistive load is half the maximum power.
- An a.c. transformer is a device for increasing or decreasing an a.c. voltage. It consists of a primary coil of Np turns and voltage Vp and secondary coil of Ns turns and voltage Vs wrapped around an iron core.
- For an ideal transformer (assuming no energy is lost), the following equation is obeyed
- Power loss in the transmission lines is minimized if the power is transmitted at high voltages (i.e. low currents) since
- The equation
[accordions autoHeight=’true’]
[accordion title=”1. Particle Nature of Light”]
- A photon is a quantum of electromagnetic radiation.
- The energy of a photon is given by E=hf, where h is Planck’s constant (6.63
[/accordion]
[accordion title=”1.1 Photoelectric Effect”]
- The photoelectric effect is the emission of electrons from a metal surface when electromagnetic radiation of sufficiently high frequency is shone on it.
- The energy of an incident photon is the sum of the maximum kinetic energy
- where
- The work function
[/accordion]
[accordion title=”1.2 Line Spectra”]
- An atom is in the ground state when its electron occupies the lowest energy level. When the atom gains energy, its ground state electron makes a transition to a higher energy level. The atom is said to be in an excited state.
- At this excited state, the electron is unstable. It will jump to a lower energy level by emitting a photon whose energy is equal to the energy difference between the two levels. The photon energy is given hf = Ehigher – Elower.
- The emission line spectra are the spectra of light radiated by individual atoms in a hot gas when the electrons in the atoms jump from higher energy levels to lower energy levels. Each spectrum consists of coloured lines on a dark background.
- The absorption line spectra consists of dark lines on a coloured background. When a beam of white light is passed through a cool gas, photons whose energies are equal to the excitation energies of the gas atoms, are absorbed. These photons are re-emitted in all directions, so the intensity of these wavelengths in the transmitted white light beam is reduced.
[/accordion]
[accordion title=”2. Wave Nature of Particles”]
- Louis de Broglie postulated that, because photons have wave and particle characteristics, perhaps all forms of matter have both properties. Electron diffraction provides evidence for the wave nature of particles.
- The de Broglie wavelength of a particle is given by
[/accordion]
[accordion title=”3. X-ray Spectrum”]
[/accordion]
[accordion title=”4. Heisenberg Uncertainty Principle”]
[/accordion]
[accordion title=”5. Wave Function and Probability”]
- An electron can be described by a wave function
[/accordion]
[accordion title=”6. Quantum Tunneling”]
- Classically, an electron of energy E approaching a potential barrier, whose height U is greater than E, cannot penetrate the barrier but would simply be reflected and return in the opposite direction.
- However, quantum mechanics predicts that since
- The transmission coefficient T represents the probability with which an approaching electron will penetrate to the other side of the barrier. The transmission coefficient T is given by
[/accordion]
[/accordions]
The Nucleus
- existence and size demonstrated using the Rutherford
- consists of nucleons (protons and neutrons)
- isotopes of an element share the same number of protons but different number of neutrons.
Nuclear Reactions
- nuclear reactions involve two or more reactants.
- represented using the form:
- for a reaction that releases energy, mass-energy of reactants = mass-energy of products + E,
where - binding energy is the energy released when the nucleus is formed from its separate protons and neutrons. The same amount of energy is required to break up a nucleus into its constituent nucleons.
- binding energy per nucleon (
- nuclear fission is the disintegration of a heavy nucleus into two lighter nuclei of comparable mass with the emission of neutrons and release of energy.
e.g. - nuclear fusion occurs when two light nuclei combine to form a single more massive nucleus, leading to the release of energy.
e.g.
- The following quantities are always conserved:
- proton number & neutron number
- momentum
- mass-energy
Radioactive Decay
- spontaneous and random emission of radiation from a radioactive nucleus.
http://youtu.be/Qlb5Z8QBpcI
where A is the rate of disintegration or activity, N is the number of radioactive nuclei and
where x could represent the activity, number of undecayed particles or received count rate.- half-life (
- From
- You may also use
[accordions autoHeight=’true’]
[accordion title=”1. Definitions”]
- The magnetic flux density at a point is defined as the force acting per unit current per unit length of the conductor when the conductor is placed at right angles to the field.
- One tesla is the uniform magnetic flux density which, acting normally to a long straight wire carrying a current of 1 ampere, causes a force per unit length of 1 N m–1 on the conductor.
[/accordion]
[accordion title=”2. Magnetic Fields”]
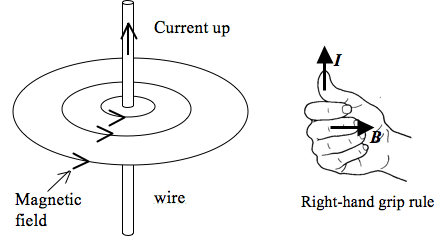
- The following are the vector symbols used in diagrams to represent the direction of vectors in 3 dimensional space:
- The following are some important points to take note when representing a magnetic field by magnetic field lines:
- Magnetic field lines appear to originate from the north pole and end on the south pole.
- Magnetic field lines are smooth curves.
- Magnetic field lines never touch or cross.
- The strength of the magnetic field is indicated by the distance between the lines – closer lines mean a stronger field.
[/accordion]
[accordion title=”3. Force on a Current-Carrying Conductor in a Magnetic Field”]
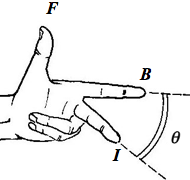
- When a wire of length
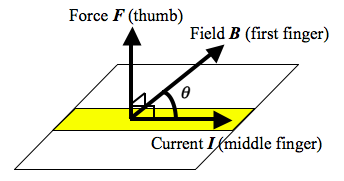
- The directions of the vectors can be recalled by using the Fleming’s Left-Hand Rule.
[/accordion]
[accordion title=”4. Force on a Moving Charge in a Magnetic Field”]
- A charge
[/accordion]
[accordion title=”5. Magnetic fields of current-carrying conductors”]
[/accordion]
[accordion title=”6. Ferromagnetic Materials”]
[/accordion]
[accordion title=”7. Force between Two Parallel Current-Carrying Conductors”]
- Like currents attract and unlike currents repel.
[/accordion]
[/accordions]
Types of Forces
- Static friction
- Frictional force between surfaces at rest with respect to each other.
- It increases with increasing applied force up to a maximum value (which is proportional to normal contact force).
- Kinetic friction
- Frictional force acting between surfaces in relative motion.
- Viscous forces
- Resistive force experienced by a solid moving in a fluid.
- Dependent on speed of object v, e.g.
[accordions autoHeight='true']
[accordion title=”1. Newton’s Laws of Motion”]
- Newton’s First Law: a body will remain in its state of rest or uniform motion in a straight line unless acted upon by a resultant force.
- Newton’s Second Law: the rate of change of momentum of a body is proportional to the resultant force acting on it and the change takes place in the direction of the resultant force.
- Newton’s Third Law: if body A exerts a force on body B, then body B exerts an equal and opposite force on body A
[/accordion]
[accordion title=”2. Linear Momentum”]
- The linear momentum of a body is defined as the product of its mass and its velocity.
- Impulse is the product of the force acting on a body and the time interval during which the force is exerted. It is equal to the change in momentum of the body.
- For constant force, impulse =
- In general, impulse =
- For constant force, impulse =
[/accordion]
[accordion title=”3. Collision Problems”]
- The principle of conservation of momentum states that the total momentum of a system of colliding objects remains constant provided no resultant external force acts on the system.
- Conservation of momentum applies to both elastic and inelastic collisions.
- Conservation of kinetic energy applies only to elastic collisions.
- Relative speed of approach = Relative speed of separation
[/accordion]
[/accordions]