I created a new GeoGebra app based on an ideal Stirling Cycle (A. Romanelli Alternative thermodynamic cycle for the Stirling machine, American Journal of Physics 85, 926 (2017)) which includes two isothermal and two isochoric processes. The Stirling engine is a very good example to apply the First Law of Thermodynamics to, as the amount of gas is fixed so the macro-variables are only pressure, temperature and volume. Simplifying the cycle makes it even easier for first time learners to understand how the engine works.
For those who prefer to be impressed by an actual working model, it can be bought for less than S$30 on Lazada. All you need for it to run is a little hot water or some ice. Here’s a video of the one I bought:
The parts of the Stirling engine are labelled here:
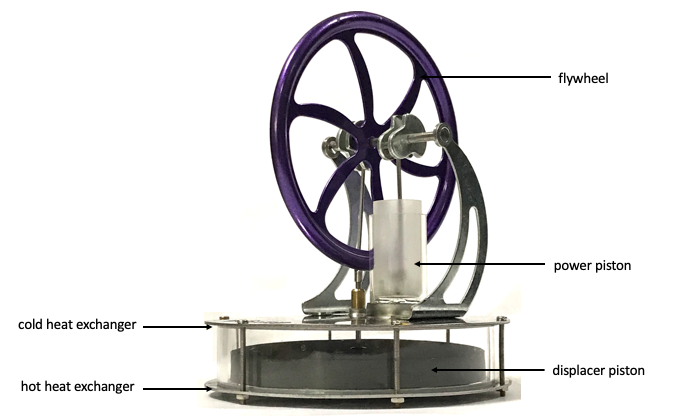
My simulation may not look identical to the engine shown but it does have the same power piston (to do work on the flywheel) and displacer piston (to shunt the air to and fro for more efficient heat exchange).
Geogebra link: https://www.geogebra.org/m/pbnw2yas